Description of the scientific activities
DESCRIPTION OF THE SCIENTIFIC ACTIVITIES
The Novel Radiopharmaceuticals research group is committed to advancing the field of targeted, personalized medicine through the design and synthesis of innovative diagnostic and therapeutic molecular radiopharmaceuticals. Our research focuses on several key areas:
- Radionuclide production - our research explores innovative production routes for both established and emerging radioisotopes. Utilizing the advanced facilities of the Maria research reactor and the newly installed IBA Cyclone XP 30 cyclotron at the CERAD Centre within NCBJ, we aim to enhance the efficiency and scalability of radionuclide production. This includes optimizing processes for both commercial and novel isotopes to meet the growing demands of nuclear medicine.
- Target material preparation and irradiation - we focus on the preparation of target materials in various forms, such as very thin layers electrodeposited or vaporized on a support, thin foils, powders, or solutions. Effective radiochemical separation is crucial, requiring not only high recovery rates of the produced radioisotopes but also efficient recovery of the target materials, which are often isotopically enriched and thus costly.
- Biomolecule labelling - we focus on the precise labelling of biomolecules, such as monoclonal antibodies, their fragments, and peptides with various radionuclides. This is achieved using chelators or prosthetic groups, ensuring targeted accuracy for both diagnostic and therapeutic purposes.

Modification of octreotide via disulfide rebridging - Multimodal nanomaterials development - our research extends to creating advanced multimodal nanomaterials tailored for dual diagnostic and therapeutic applications in medicine. Some of these nanomaterials are engineered to possess luminescent properties upon exposure to Near Infrared Radiation (NIR) and to incorporate radioisotopes, allowing for dual-mode detection of cancerous cells, such as those in lymph nodes. Together with scientists from CEA are performed studies on the design and synthesis of theranostic micellar nanocarriers for imaging and targeted radiosensitization due to encapsulation of gold nanoparticles in micelles and their simultaneous labelling with theranostic lutetium-177 radioisotope.

Biomedical applications of up-converting nanoparticles (UCNPs)
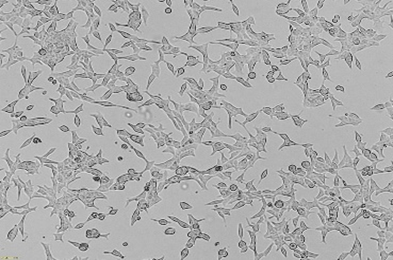
Nanomicellar system for imaging and targeted radiosensitization - Preclinical evaluation - we conduct comprehensive evaluations, including both in vitro and in vivo studies, to assess the efficacy and safety of the developed compounds and materials. This rigorous testing is essential for validating the potential of new radiopharmaceuticals before they advance to clinical trials.
The Novel Radiopharmaceuticals group benefits from access to unique research infrastructure, unparalleled on a national and European scale. This includes facilities for radionuclide production via reactor and cyclotron, alongside fully equipped chemical, radiochemical, and biological laboratories. To achieve our ambitious goals, we plan to leverage our state-of-the-art infrastructure and invest in advanced new equipment, supported by recently awarded funding. Expansion strategy includes enhancing our preclinical laboratory with modern SPECT-CT and PET-CT imaging systems, which will be in the near future integrated with optical imaging technologies such as fluorescence and bioluminescence tomography, Cherenkov imaging, and magnetic resonance imaging. Additionally, we aim to upgrade or replace current equipment, including multimodal plate readers (featuring fluorescence, chemiluminescence, and autoradiography modules), flow and image cytometers with updated lasers and filters, multi-channel gamma and beta scintillation counters, and fluorescence microscopes. These investments in cutting-edge technology will empower us to conduct a comprehensive range of studies on novel radiopharmaceuticals and their preclinical evaluation, demonstrating both diagnostic potential and therapeutic efficacy.
We are excited to build collaborations with other research groups and industry partners to expand our capacity for participating in European projects within large consortia and to offer specialized research services to industrial partners. By joining forces, we can drive innovation and make meaningful contributions to the field of radiopharmaceuticals.
As we expand our research infrastructure, we are also opening new opportunities for talented professionals. We will soon be recruiting specialists in cyclotron operation and SPECT-PET-CT imaging systems. Additionally, the grants we have received will allow us to welcome ambitious and passionate scientists who are eager to work in an international team and collaborate with leading experts in the field.
Our strategic collaborations with esteemed partners such as CEA and VTT are pivotal to our research. These partnerships facilitate the development of innovative targeting biomolecules, including those produced via phage-display technology and synthesized peptides. Our close collaboration with the Radioisotope Centre POLATOM (NCBJ) allows for rigorous preclinical evaluations, further supporting the translation of our research into clinical applications and routine production.
With these extensive resources and collaborative efforts, the Novel Radiopharmaceuticals research group is poised to drive significant advancements in personalized medicine.